Experience the groundbreaking DragonFly™ Transcatheter Mitral Valve Clip System by Valgen Medtech. Discover its success in treating severe mitral regurgitation. Find out more!



Recently, the heart valve team at Qilu Hospital of Shandong University successfully performed two high-difficulty edge-to-edge mitral valve repair surgeries using the DragonFly Transcatheter Mitral Valve Clip System, independently developed by Valgen Medtech. These surgeries treated two patients with severe mitral regurgitation complicated by chordal rupture, marking the first implants of DragonFly in this region since its launch.
The surgeries were guided by Professor Zhang Cheng and carried out by a team led by Professors An Guipeng, Dong Mei, and Liu Xiangjuan from the Cardiology Department. Post-treatment, both patients' mitral regurgitation levels were reduced to mild, and their heart function improved. In the long run, their quality of life is expected to gradually improve.
In November 2023, Hangzhou Valgen Medtech Co., Ltd.'s DragonFly™ Transcatheter Mitral Valve Clip System (hereinafter referred to as "DragonFly™") received official approval from the National Medical Products Administration (NMPA), making it the first domestically approved transfemoral mitral valve clip product globally.
Surgical Team in Action
In February 2024, the DragonFly™ Transcatheter Mitral Valve Clip System successfully completed the first confirmatory clinical enrollment in Europe at Universitätsklinikum Bonn (University Hospital Bonn) in Germany. This milestone follows its NMPA approval, pioneering the use of Chinese transcatheter mitral regurgitation treatment products in Europe.
Patient One:
A 62-year-old male was admitted due to 12 years of chest tightness and shortness of breath, which worsened over the past two months. His medical history included a stroke and previous coronary artery bypass grafting and mitral valve repair for coronary artery disease, myocardial infarction, and mitral insufficiency. Echocardiography indicated extensive myocardial ischemia with heart failure and severe mitral regurgitation with mild anterior leaflet prolapse. The anterior leaflet measured 2.4 cm, the posterior leaflet 1.3 cm, and the valve orifice area 6.87 cm², with a lesion width of 1.6 cm.
Treatment Challenges:
1. Mixed severe mitral regurgitation with A2 prolapse and chordal rupture made mitral clip capture difficult.
2. Left heart system enlargement and annulus dilation required careful monitoring of leaflet tension to prevent perforation or tearing.
Treatment Plan:
Considering the regurgitation width, the team used a wide model mitral clip to address the prolapse and chordal rupture. A transseptal puncture was made posteriorly at a height of 4.11 cm, and a clip was implanted in zone 2, successfully closing the prolapsed area. Postoperative results showed a transvalvular pressure gradient of 2 mmHg and disappearance of pulmonary vein regurgitation, with MR reduced to 1+.
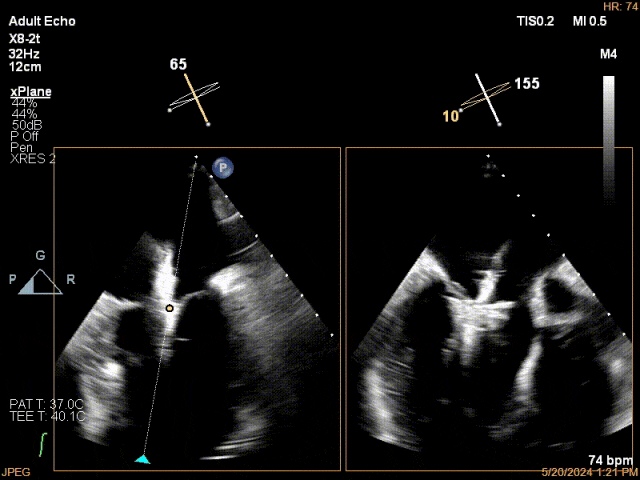
Mitral Leaflet Capture
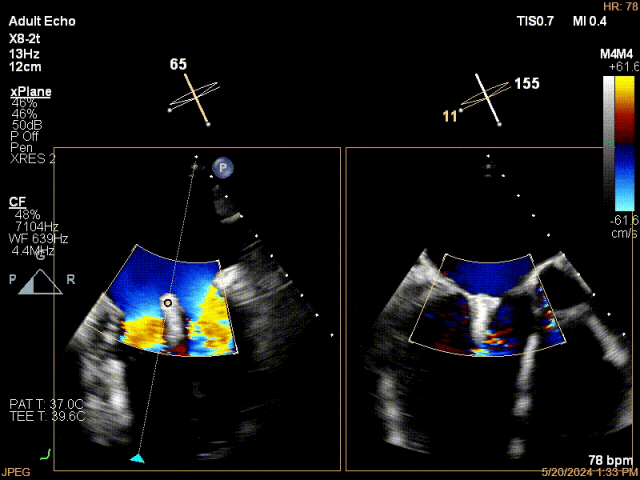
Mitral Clip Release
Patient Two:
A 65-year-old female was admitted due to chest tightness and coughing for two months. Echocardiography showed global heart enlargement, reduced left ventricular diastolic function, and severe mitral regurgitation with chordal rupture in the P3 and C2 regions, causing prolapse with flail motion. The anterior leaflet measured 1.9 cm, the posterior leaflet 1.0 cm, and the valve orifice area 6.6 cm², with a prolapse width of 1.1 cm and height of 1.2 cm.
Treatment Challenges:
1. The lesion involved the commissural area, making mitral clip operation challenging. Constant monitoring was required to avoid hooking leaflets or chordae during positioning and capture.
2. The high prolapse height of 1.2 cm made simultaneous anterior and posterior leaflet capture difficult, necessitating adjustments for single-side capture.
Treatment Plan:
The team made multiple adjustments during surgery, and after successfully capturing the anterior and posterior leaflets, implanted a clip in the zone 3 near the C2 commissure. Postoperative results showed a transvalvular pressure gradient of 2 mmHg and regurgitation reduced to 1+, with immediate significant improvement.
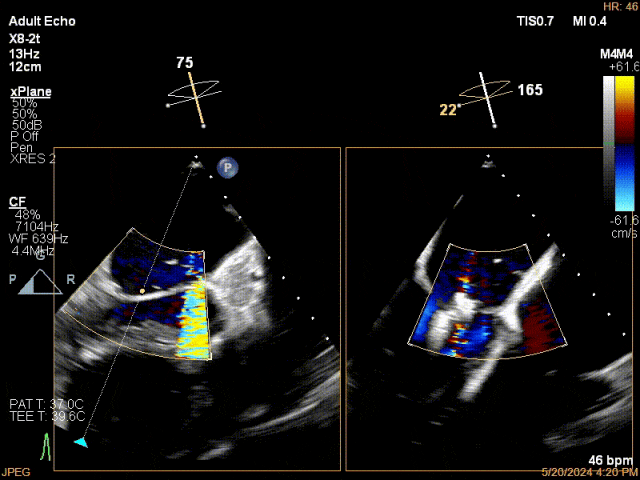
Mitral Clip Release
Postoperative evaluation by Professor An Guipeng: "The key to successful surgery lies in the precise control of the device during the procedure, ensuring optimal leaflet coaptation and stable clip attachment to ensure safety and effectiveness. The successful implantation of the DragonFly™ system at our hospital has increased our confidence and anticipation for the product, hoping it will benefit more patients under our care."
DragonFly™ was developed by Valgen Medtech in collaboration with the team of Academician Wang Jian'an from the Second Affiliated Hospital of Zhejiang University School of Medicine and the team of Academician Zhang Xingdong from the National Engineering Research Center for Biomaterials at Sichuan University.

Image Source: Valgen Medtech Official Website
The product consists of a guiding sheath and a mitral valve clip system, which includes the clip and a delivery system. The clip's elastic central sealing mesh structure enhances sealing and reduces residual regurgitation, while lowering leaflet capture force. Additionally, the clip's independent leaflet capture and repositioning functions improve precision and reduce the risk of clip detachment and leaflet perforation.
The product is suitable for patients with degenerative mitral regurgitation (MR≥3+) assessed by the cardiac team to be at high risk for surgery and with appropriate mitral valve anatomy. The launch of this product offers more options for clinical treatment.
The DragonFly™ Transcatheter Mitral Valve Clip System features four innovative designs:
1. An extendable central sealing mesh with infinitely adjustable locking arms ensures stable leaflet capture, reduces leaflet tension, minimizes damage, and decreases residual central regurgitation and postoperative transvalvular pressure gradient.
2. Four clip sizes accommodate different leaflet anatomies, providing more options for various mitral regurgitation conditions.
3. Independent leaflet capture for single-side capture improves success rates and efficiency in complex cases.
4. A precision-controlled delivery system with a visual, jointed, graduated operation system and unique three-stage adjustable catheter design enhances operational accuracy and convenience.
DragonFly™ is the first domestically approved transfemoral mitral valve clip system in China, receiving NMPA approval in November 2023.

NMPA approval for transfemoral mitral valve clip system
Since its market debut on January 25, 2024, DragonFly™ has been implanted in regions including Zhejiang, Henan, and Xi'an, with ongoing expansion to new medical institutions.
DragonFly™ holds independent intellectual property rights. By September 2023, over 150 domestic and international patents have been applied for, with 95 invention patents, 28 PCT applications, and 30 granted patents.
Study Design:
A prospective, single-arm, multicenter clinical study was conducted to evaluate the safety and effectiveness of the DragonFly™ system in treating symptomatic patients with MR≥3+ and high surgical risk DMR. The primary endpoint was a 12-month treatment success rate, defined as no all-cause mortality, no mitral valve dysfunction requiring surgery, and no MR>2+ at 12 months postoperatively.
Baseline Information:
From May 2021 to January 2022, 120 patients were enrolled and treated across 27 centers in China, with the last follow-up completed in December 2022. The average age of participants was 74.9±5.7 years, with 49.2% female, 39.2% having coronary artery disease, 18.3% with a history of cardiovascular intervention or surgery, and 70.8% with chronic obstructive pulmonary disease.
Trial Results:
The 12-month treatment success rate was 87.5%. The DragonFly™ system demonstrated significant therapeutic performance, achieving the primary efficacy endpoint.

Primary Efficacy Endpoint(Composite) at 12 months
The proportion of patients with MR≤2+ was 90.4% at one month and 92.0% at one year, showcasing the durability of the DragonFly™ system in correcting DMR.
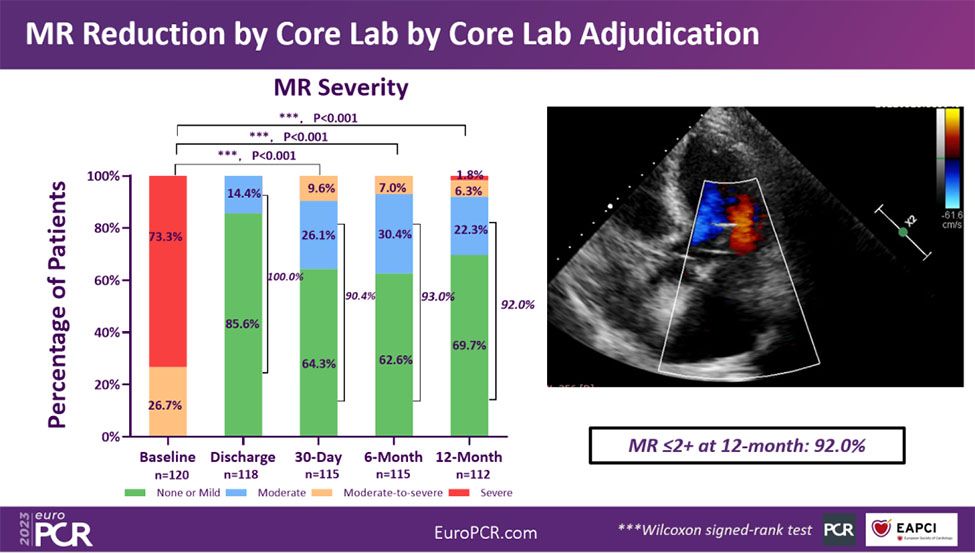
MR Reduction by Core Lab by Core Lab Adjudication
Immediate postoperative and 12-month procedure success rates and device implantation success rates were 99.2%. During surgery, 52.5% of patients received one clip, and 42.5% received two clips.
The average procedural time was 116.67±51.26 minutes, with an average device operation time of 96.58±47.70 minutes and average fluoroscopy time of 34.05±20.17 minutes. Immediate and 12-month postoperative average mitral transvalvular pressure gradients (TMPG) were 2.88±1.34 mmHg and 3.19±1.38 mmHg, respectively, further indicating good device operability.
Twelve-month follow-up results showed significant improvement in patients' heart function and quality of life, with the proportion of NYHA class I/II patients increasing from 32.4% at baseline to 93.6% at 12 months, and the Kansas City Cardiomyopathy Questionnaire (KCCQ) score improving by 30.64±18.35 points from baseline.
Conclusion:
The DRAGONFLY-DMR trial results indicate that the DragonFly™ Transcatheter Mitral Valve Clip System is safe, effective, and highly operable, providing a viable treatment option for high-surgical-risk degenerative mitral regurgitation patients.

Valgen Medtech
Established in 2015, Hangzhou Valgen Medtech Co., Ltd. focuses on interventional treatment products and technologies for mitral and tricuspid valve diseases. The company has applied for over 500 patents globally, with more than 150 authorized.
Three innovative products independently developed by the company have passed the NMPA's special review process for innovative medical devices. The Dragon series, representing a variety of interventional treatment devices, will further enrich clinical options for structural heart disease treatment in China.
The core product, DragonFly™ Transcatheter Mitral Valve Clip System, is the first Chinese-developed edge-to-edge repair product approved for the transfemoral approach, receiving NMPA approval for market entry in China. The product is supported by the national "14th Five-Year Plan" key research and development project.
Other marketed products include Pu Jie Jie™ medical radiation protection screens, Magpie™ balloon dilatation catheter, DragonPath™ transseptal puncture system, and Firework™ hard guidewire series.