The next-generation absorbable magnesium alloy stent Freesolve arrives in China, offering improved vascular support, safety, and complete absorption within 12 months.


Recently, the next-generation magnesium alloy absorbable coronary stent Freesolve, developed by the German cardiovascular company Biotronik, was successfully introduced at Hainan Hospital, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Boao Research Hospital, Hainan).
The teams led by Professor Zhang Ruiyan from Ruijin Hospital, Professor Lu Chengzhi from Tianjin First Central Hospital, and Professor Wang Sheng from Hainan Provincial People's Hospital successfully performed the first clinical application of the Freesolve magnesium alloy absorbable stent at Ruijin Hainan Hospital. The success of the surgery not only marks Ruijin Hainan Hospital’s leadership in the field of absorbable stent implantation but also takes a significant step forward in making this innovative device accessible to more Chinese patients.
Biotronik’s self-developed Freesolve magnesium alloy absorbable coronary stent was officially CE certified and launched in February 2024. Benefiting from the "early trial and implementation" special policy in the Lecheng Pioneer Zone, the Freesolve magnesium alloy absorbable stent submitted its application immediately after receiving CE approval and was granted permission by the Hainan Provincial Drug Administration for clinical use in the Lecheng Pioneer Zone.
On the same day, the product launch event for the Freesolve magnesium alloy absorbable stent was successfully held at Ruijin Hainan Hospital.
In 2016, Biotronik launched the second-generation absorbable magnesium alloy stent Magmaris®, which became the world’s first commercially available absorbable metal stent. Building on the excellent clinical performance of the Magmaris® RMS, Biotronik further optimized it, leading to the creation of the third-generation Freesolve™ RMS.
Freesolve™ RMS continues the technology of Magmaris® RMS with several technical improvements to meet the needs of interventional cardiologists and achieve optimal patient treatment outcomes. It aims to provide higher-quality stent treatment options for patients with coronary artery disease.

▲ Product Launch Event
Compared to previous generations, this third-generation RMS has been designed for optimal vascular support while achieving magnesium absorption within 12 months. According to Biotronik's BIOMAG-I clinical study, the Freesolve stent achieves 99.3% absorption within 12 months after implantation, with consistently stable performance regardless of lesion characteristics, and restores vascular motility.
Freesolve™ RMS uses Biotronik’s patented magnesium alloy BIOmag® as the stent scaffold, combining high mechanical performance with excellent biocompatibility. Additionally, Freesolve™ RMS offers a wider range of sizes to meet the treatment needs of patients with different vascular anatomies.
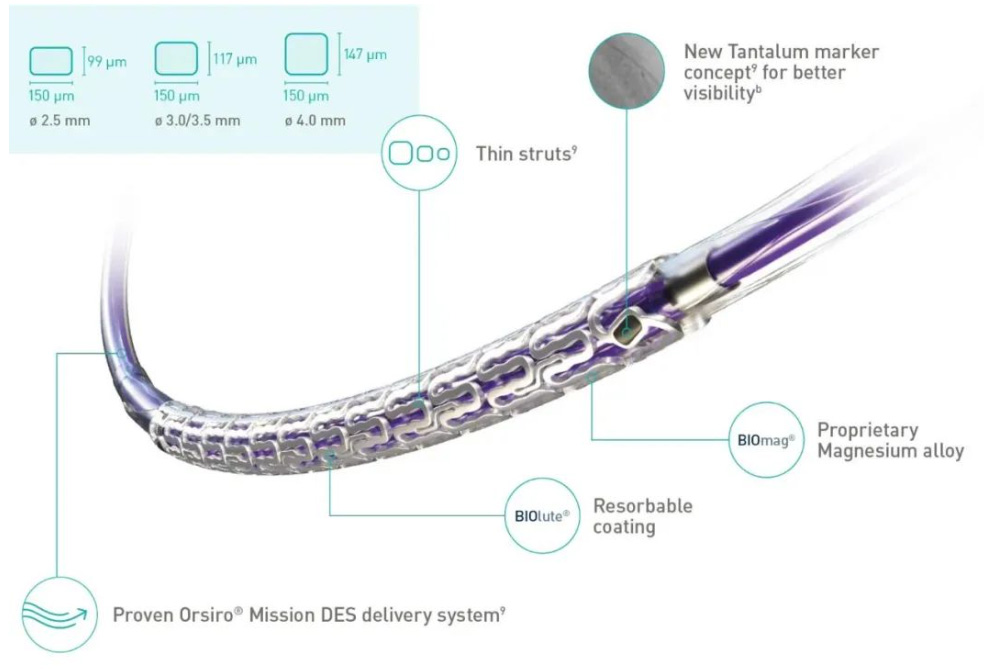
▲ Freesolve™ Absorbable Magnesium Stent
Freesolve™ adopts an open-cell design with six crowns and two connecting rings. Compared to the first and second-generation magnesium alloy absorbable coronary stents, Freesolve™ offers better trackability, deliverability, and fluid dynamics, thanks to its electrochemically polished smooth edges. The stent also has two radiopaque markers for positioning during imaging, which are coated with silicon to prevent electrochemical reactions with the magnesium alloy. It also uses the same biodegradable polylactic acid (PLLA) coating as the Orsiro stent, which contains sirolimus. This coating has a prolonged degradation process, lasting more than 24 months.
Improved Deliverability and Visibility: Freesolve™ RMS has thinner stent struts compared to previous generations and uses Biotronik’s latest DES Orsiro® Mission delivery system. A new marker enhances visibility under X-ray.
Optimized Vascular Support: Freesolve™ RMS is made from Biotronik’s patented BIOmag® magnesium alloy, providing better mechanical properties and longer-lasting radial support.
Complete Absorption within 12 Months: Freesolve™ RMS achieves a 99.3% stent strut degradation rate within 12 months after implantation, while synchronously completing vascular repair.
More Size Options: Stent diameters range from 2.5-4.0 mm, and lengths range from 13-30 mm.
Outstanding Safety and Efficacy: One-year follow-up data from the BIOMAG-I study shows Freesolve™ RMS achieved a low target lesion failure rate (2.6%), low clinically driven target lesion revascularization rate (2.6%), with no myocardial infarction or definite/probable stent thrombosis.
At the 2024 European Course on Revascularization (EuroPCR 2024), the latest 24-month follow-up results from the BIOMAG-I trial of the Freesolve stent were presented, demonstrating excellent clinical performance and safety.
Study Design
The BIOMAG-I trial is a prospective, multicenter, non-randomized first-in-human study, including patients with primary coronary lesions from 14 centers in 8 European countries. Clinical follow-up was conducted at 1, 6, and 12 months post-surgery, as well as at 2, 3, 4, and 5 years. The primary endpoint was late lumen loss (LLL) at 6 months, while secondary endpoints included segmental LLL, target lesion failure (TLF), target lesion revascularization (TLR), and definite/probable stent thrombosis.
Study Results
A total of 116 patients with 117 primary coronary lesions were enrolled in the study. By the 24-month follow-up, 115 patients had completed clinical follow-up, with a compliance rate of 99.1%.
At the 24-month follow-up, the TLF rate was 3.5%, the TLR rate was 3.5%, with no cardiac deaths, target-vessel-related myocardial infarction, or definite/probable stent thrombosis events.
The BIOMAG-I first-in-human study demonstrated the restoration of vascular vasomotor function in patients treated with the Freesolve stent. OCT analysis showed that 99.3% of the stent was fully degraded within one year, with favorable late lumen loss (LLL) results at 6 and 12 months.
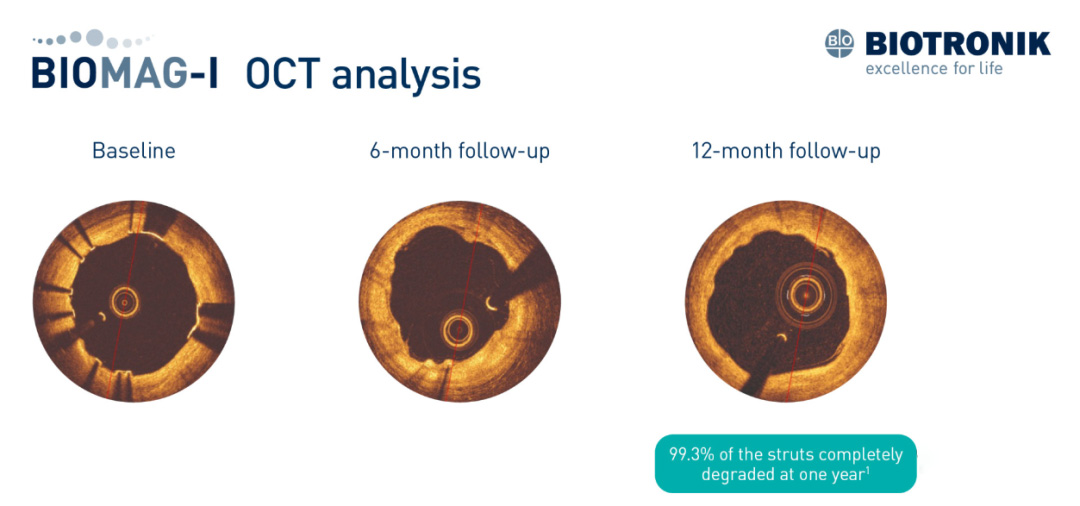
▲ OCT analysis
To further expand the evidence base behind the Freesolve RMS, Biotronik launched the BIOMAG-II study in Q2 2024, comparing the Freesolve RMS with contemporary drug-eluting stents (DES). The first patient was enrolled on May 13. The study plans to recruit 1,859 patients with primary coronary artery stenosis, with the primary endpoint being target lesion failure (TLF) at 12 months.
Coronary artery disease is one of the most common cardiovascular diseases and one of the leading causes of death worldwide. According to the "China Cardiovascular Health and Disease Report 2019," the prevalence of cardiovascular disease in China is steadily rising, with an estimated 330 million people affected, including 11 million cases of coronary heart disease.
At the same time, cardiovascular disease is the leading cause of death in China, accounting for 45.50% of all deaths in rural areas and 43.16% in urban areas. Two out of every five deaths are due to cardiovascular disease.
Currently, the primary treatment for coronary heart disease is intravascular intervention, which involves using balloon catheters and drug-eluting stents (DES) to widen narrowed or blocked arteries and restore blood flow. Since DES materials commonly include cobalt-chromium alloys, the stent remains permanently implanted in the coronary arteries, leading to risks such as stent thrombosis and impaired vascular elasticity and motion post-surgery.
In addition, the presence of metallic foreign bodies in the coronary arteries requires patients to take antiplatelet drugs for life, increasing the risk of bleeding and imposing a financial burden on patients.
The clinical application of absorbable magnesium alloy stents effectively avoids the permanent restriction of vascular elasticity caused by DES, as well as the long-term presence of metal foreign bodies in the patient's body, realizing intervention without implantation.
According to a 2023-2029 QYResearch report, the global market for absorbable polymer stents was valued at approximately $631 million in 2023 and is expected to reach $1.04 billion by 2029, with a compound annual growth rate (CAGR) of 8.7%.
Bare-metal stents were the first to be used clinically, but their metal-wire foreign-body response often led to neointimal hyperplasia at the lesion site, increasing the risk of restenosis by 30%-40%. Moreover, since the stent remains permanently in the artery, it continuously restricts normal vascular motion and may trigger long-term, persistent inflammation.
Drug-eluting stents, which add a drug coating to bare-metal stents, reduce the probability of restenosis to about 5% by inhibiting neointimal hyperplasia in the coronary arteries. However, the drug coating may interfere with blood coagulation, increasing the risk of thrombus formation, which can lead to myocardial infarction or stroke.
As interventional treatment techniques for coronary artery disease continue to improve, "bioabsorbable stents" are providing patients with another high-quality treatment option. Both domestic and international manufacturers are entering this market.
Globally, the main manufacturers of absorbable polymer stents include Abbott Vascular, Boston Scientific, Medtronic, Kyoto Medical Planning, and Lepu Medical, with the top five companies holding approximately 73.1% of the market share. Currently, the core global manufacturers are concentrated in the United States.
In terms of product type, polylactic acid currently dominates, accounting for about 74.6% of the market share. Hospitals are the main source of demand, making up approximately 72.5% of the market.
Moreover, as the traditional coronary stent market faces the impact of centralized procurement, finding new growth areas has become a necessity for stent companies. Absorbable stents present a promising opportunity in this regard.
For example, Lepu Medical's absorbable polymer stent NeoVas. In 2019, Lepu Medical's self-developed degradable stent NeoVas obtained China’s first national certificate in this field. In 2021, despite the sharp decline in the performance of traditional stents, Lepu Medical achieved a year-on-year revenue growth of 827.36%, driven by an innovative portfolio of interventional products, including degradable stents, cutting balloons, and drug balloons.
NeoVas provides mechanical support comparable to metal stents after implantation. It begins to degrade after about one year and is fully absorbed as water and carbon dioxide by the body within three years, without affecting future treatments or imaging examinations (MRI/CT). Furthermore, the surface of the NeoVas stent is coated with the antiproliferative drug sirolimus, which effectively prevents restenosis at the lesion site, helping to achieve optimal clinical outcomes.
From the perspective of coronary stent technology and market development, drug-eluting stents will remain the mainstream in the short term, while degradable stents are expected to expand their market share. In terms of stent platforms, thin-walled stents can significantly reduce the risk of thrombosis, and absorbable stents offer more long-term benefits to patients. From the standpoint of drug-coating technology, future developments aim to reduce drug dosages, improve drug utilization rates, and use techniques such as single-sided coatings, endothelialization promotion, degradable or no coatings to reduce restenosis and promote endothelial repair.