Learn how stringent manufacturer quality control ensures that nerve block needles, including those that are echogenic and ultrasound-guided, are safe, accurate, and dependable.


In modern medical practice, precision and reliability are non-negotiable — especially when it comes to anesthesia delivery. One critical tool in this field is the nerve block needle, designed to deliver local anesthetics accurately near targeted nerves, reducing pain during surgery and other procedures.
The quality of the needle itself is just as important to the success of a nerve block as the anesthesiologist's skill. These needles satisfy medical safety and performance standards thanks to strict quality control, high manufacturing standards, and regular product testing. Let's examine how healthcare providers can select the best products for their requirements and how manufacturer quality control ensures dependability.
The Importance of Reliable Nerve Block Needles
Precision for Patient Safety
Millimeters are important when doing a nerve block. Inaccurate placement, decreased efficacy, or even complications could result from a poorly made needle. Manufacturers can ensure sterility, tip sharpness, and dimensional accuracy by adhering to strict quality control procedures.
Durability and Consistency
Needles that bend, dull quickly, or have inconsistent lumen diameters can compromise outcomes. Quality control ensures every nerve block needle that leaves the factory meets the same high standard, reducing the risk of procedural errors.
How Manufacturer Quality Control Works
Step 1 – Material Selection and Inspection
Medical-grade alloys or premium stainless steel are chosen for their corrosion resistance, biocompatibility, and longevity. Raw materials are examined for microstructure, chemical makeup, and tensile strength prior to production starting.
Step 2 – Precision Manufacturing
Advanced manufacturing methods such as CNC grinding, laser cutting, and micro-polishing are used to produce the precise geometry required for different needle designs. This is especially important for specialized models like the Echogenic Needle For Nerve Block, which relies on surface texture to enhance ultrasound visibility.
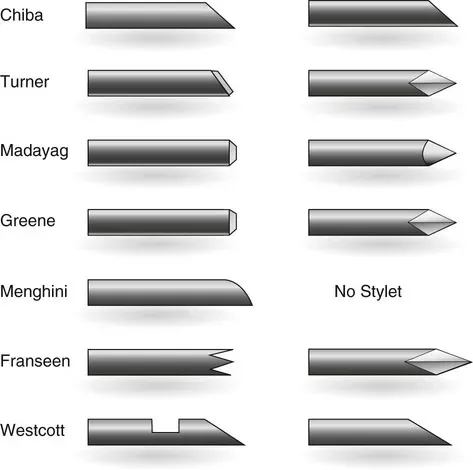
Step 3 – Tip Geometry Verification
Needle tip designs vary depending on clinical application — for example:
Disposable Medical Ultrasound Nerve Block Needle: Features an optimized bevel for minimal tissue trauma.
Echo Needle For Nerve Block:
Designed to enhance procedural safety by being easily visible under ultrasonography.
Manufacturers check bevel angles and tip sharpness using laser measurement instruments and microscopes.
Step 4 – Lumen and Gauge Accuracy
For consistent anesthetic flow and compatibility with syringes or catheters, the gauge size needs to be precise. For products like the 20G Ultrasound Nerve Stimulation Needle to function consistently, extremely strict tolerances are needed.
Step 5 – Sterilization and Packaging
Without adequate sterilization, even the best-made needle can be dangerous. Manufacturers frequently use ethylene oxide (EtO) or gamma radiation sterilization in accordance with ISO 13485 and CE-certified procedures. The packaging is tested for puncture resistance and is made to remain sterile until it is used.
The Role of Testing in Quality Control
Mechanical and Stress Testing
Bend, flex, and tensile tests are conducted by manufacturers to make sure the needle won't break while being used. For instance, in a variety of clinical settings, Nerve Block Plexus Stimulation Needle models need to stay conductive and upright.
Visual and Dimensional Inspection
The absence of burrs, surface imperfections, or manufacturing flaws is guaranteed by high-resolution imaging. Every dimension is compared to design specifications using optical measurement systems.
Functional Testing
Before an item is authorized for sale, functional tests are performed on stimulation and echogenic needles to confirm electrical conduction or ultrasound visibility.
Customization and Quality Control
Meeting Unique Clinical Needs
Custom lengths, gauges, or handle designs are occasionally needed in hospitals and clinics. Significant value is added by a manufacturer who can provide customized nerve block needle solutions while upholding stringent quality control.
Examples of Custom Options:
Greater nerve access through longer shaft lengths
Improved ultrasound guidance through improved echogenic markings
Ergonomic handles to enhance clinician comfort
Why Manufacturer Reputation Matters
Track Record of Compliance
Reputable producers adhere to international quality standards such as FDA 21 CFR Part 820, ISO 13485, and CE marking. They can reliably meet strict requirements, as evidenced by their compliance history.
Transparent Quality Documentation
Reputable vendors offer test results, certificates of conformance, and batch records as evidence that each disposable medical ultrasound nerve block needle or echo needle for nerve block satisfies specifications prior to shipment.

Although cost is always a factor, using inexpensive needles without adequate quality control could lead to expensive complications or the need for repeat procedures. Purchasing high-quality goods eventually saves money and time.
Certifications – Look for ISO 13485, CE, and FDA approvals.
Product Range – Ensure the supplier offers various models, including Echogenic Needle For Nerve Block and Nerve Block Plexus Stimulation Needle.
Customization Capability – The ability to make a 20G Ultrasound Nerve Stimulation Needle to your exact requirements.
Testing Records – Ask for inspection and sterilization reports.
Reputation – Check industry reviews, client feedback, and case studies.
The foundation of safe and efficient nerve block procedures is manufacturer quality control. Every stage of the manufacturing process, from choosing the right materials to packaging it, guarantees that the nerve block needle works as intended, giving each patient accuracy, security, and dependability. Healthcare providers can be sure they are utilizing tools that adhere to the highest industry standards by selecting a supplier who places a high priority on thorough testing, compliance, and customization.