

In modern implantable medicine, the success of every precision surgery relies on medical devices whose performance is perfectly adapted to the specific application. Medical dip coating technology, like a "custom tailor for medical devices," creates unique "performance coatings" for different types of implantable medical devices through in-depth customization across four dimensions: materials, function, design, and solution. This ensures safer, more efficient, and more precise clinical applications.
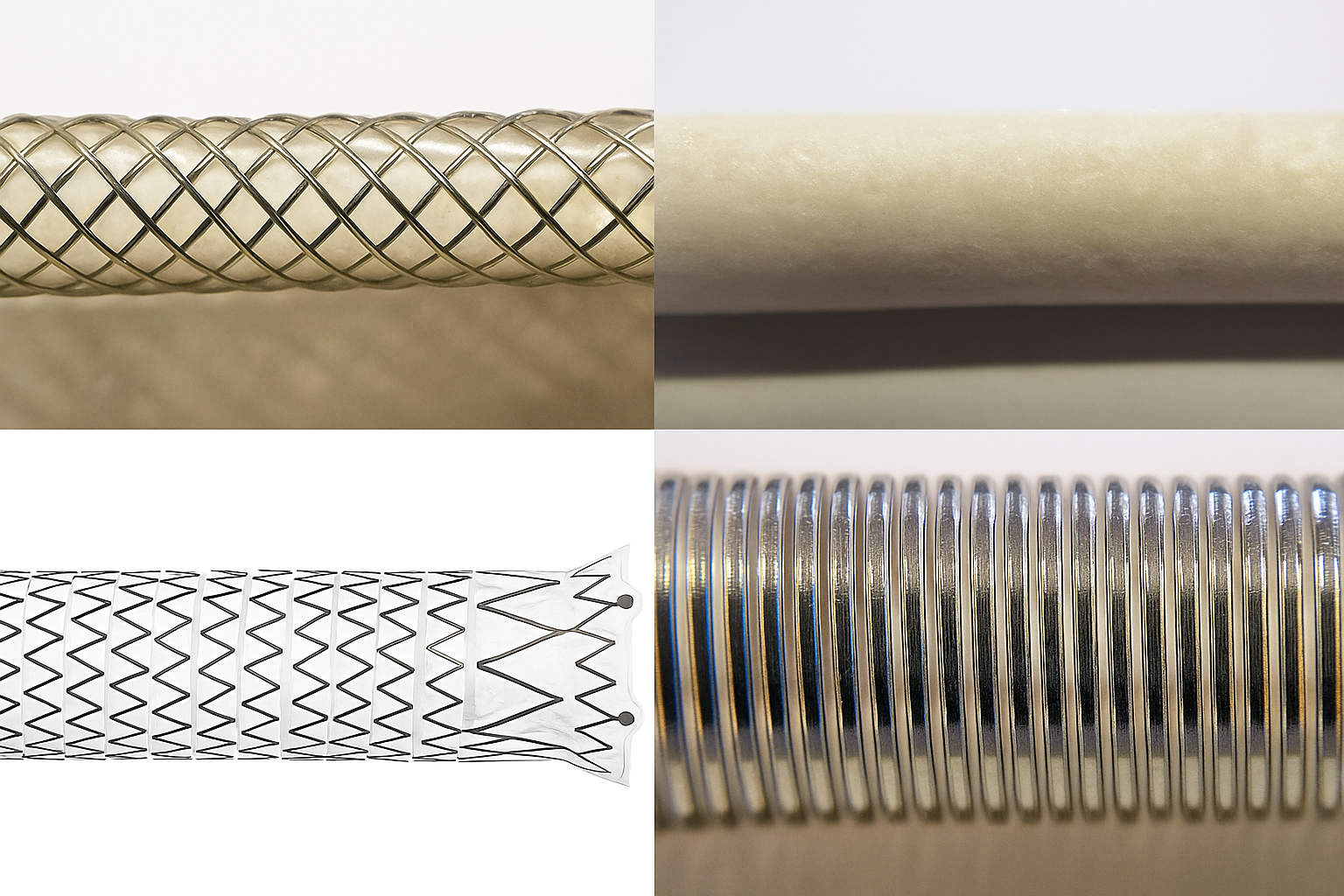
Customization Category | Compatible Materials | Core Principle | Advantages | Application Scenarios |
|---|---|---|---|---|
Biocompatibility Priority | Medical Silicone | Silicone molecular structure is stable, non-allergenic, non-toxic, and does not trigger immune rejection when contacting human tissues | High flexibility and fit, reduces friction damage to soft tissues like blood vessels and mucosa | Cardiovascular catheters, airway stents, endoscopic assist devices that require long-term or short-term contact with sensitive tissues |
Wear Resistance Priority | Polyurethane (PU) | PU material has high molecular chain density, moderate surface hardness, excellent tear and friction resistance, able to withstand mechanical wear from repeated use | Long service life, coating not prone to falling off or cracking, can withstand pulling and friction in complex interventional procedures | Orthopedic interventional guide wires, urinary catheters, frequently inserted puncture devices that require high-frequency use |
Elasticity & Fatigue Resistance Priority | Polyether Block Amide (PEBAX) | Combines rigid and flexible chain segments, can quickly return to original shape after bending and stretching, with fatigue resistance far exceeding traditional plastics | Excellent elasticity, bend resistance, can adapt to curved vascular paths, reduces device deformation during pushing procedures | Neurointerventional guide wires, peripheral vascular stents, precision devices that need to navigate complex anatomical structures |
Hardness & Stability Priority | Medical Nylon | Nylon has high molecular crystallinity, high surface hardness, strong impact and chemical corrosion resistance, with stable performance in in vivo environments | Good support capability, provides sufficient strength for devices, resistant to body fluid erosion, with no performance degradation after long-term implantation | Vascular stents (requiring vascular wall support), biliary stents, tubular devices that need to withstand internal pressure |
Functional customization is based on the basic material. By adding special ingredients or optimizing the process, the coating can have "extra capabilities" to directly solve clinical pain points and enhance the therapeutic value or safety of the device.
Function Type | Implementation Method | Core Advantage | Clinical Value | Potential Considerations |
|---|---|---|---|---|
Drug Sustained Release | Load anti-proliferative drugs (e.g., Rapamycin) or antibacterial drugs (e.g., Vancomycin) into coating materials. Achieve long-term drug release through coating degradation or drug diffusion. | High local drug concentration, minimal systemic side effects, precise action on lesion sites | Inhibit vascular restenosis after stent implantation; prevent local infection after orthopedic device implantation | Requires strict control of drug loading and release rate to avoid premature or insufficient release |
Super-Lubricating | Introduce hydrophilic groups (e.g., PEG chains) or use fluorocarbon materials on coating surface to reduce friction coefficient | Extremely low friction between device and tissue/body fluid, minimal push resistance | Smoother catheter navigation through vessels, reduced vascular wall damage; more precise guidewire manipulation, lower surgical difficulty | Coating requires strong adhesion to avoid lubricant layer detachment during pushing |
Anti-Bacterial/Infection Prevention | Add antibacterial components (e.g., nano-silver particles, zinc oxide) to coating or use antibacterial polymer materials to disrupt bacterial cell membrane structure | Inhibit bacterial growth on device surface, block "device-related infection" pathways | Reduce postoperative infection rates for long-term indwelling catheters (e.g., central venous catheters) and artificial joint prostheses | Ensure antibacterial components are non-cytotoxic and won't cause bacterial resistance with long-term use |
Radiopaque Positioning | Mix radiopaque agents (e.g., barium sulfate, gadolinium oxide) into coating to make devices clearly visible under X-ray, CT and other imaging equipment | Real-time device tracking for physicians, avoiding device displacement or mishandling | Improve surgical safety for devices requiring precise positioning (e.g., neurointerventional guidewires, peripheral vascular stents) | Radiopaque agent amount must be moderate to avoid affecting coating flexibility or increasing device weight |
The shapes of different interventional medical devices vary greatly (such as guidewires as thin as a hair, stents with mesh, and catheters with multi-lumen structures). The core of customized design is to adjust the process parameters so that the coating can "precisely cover without adding burden" and not destroy the original structure and function of the device.
Design Requirement | Process Solution | Final Effect | Applicable Device Examples | Process Challenges |
|---|---|---|---|---|
Complex Pattern Coverage | Adjust dipping speed (slow dipping ensures pattern gaps are filled) and curing temperature (graded curing prevents pattern deformation) | Coating uniformly covers bumpy patterns and mesh structures on the device surface, with no missed spots or accumulation | Guidewire handles with anti-slip patterns, mesh vascular stents, drainage catheters with multiple side holes | Requires precise control of dipping rate to avoid residual bubbles in deep patterns causing coating voids |
Differentiated Thickness Coating | Adopt "multiple dipping + local masking" process: areas needing thick coating are dipped multiple times, areas needing thin coating are protected with high-temperature resistant masking film | Coating thickness adjusted as needed on different parts of the same device (e.g., thin coating at catheter tip for flexibility, thick coating in middle for strength) | Cardiovascular catheters (tip needs flexibility, middle needs kink resistance), endoscopic biopsy forceps (thin coating on jaws for opening/closing, thick coating on shaft for rigidity) | Masking film must tightly adhere to device surface to avoid residual glue marks after curing |
Irregular Structure Adaptation | For curved, bifurcated, multi-lumen devices, use "rotational dipping" (ensures inner wall uniformity) and "local spraying + dipping combination" process | Coating covers without dead angles, consistent thickness at irregular parts (e.g., catheter bifurcations), without affecting device movement | Bifurcated vascular stents, multi-lumen drainage catheters, orthopedic instruments for joint areas | Need custom dipping fixtures based on device morphology to avoid device deformation during dipping process |
Customized solutions are designed for "new devices" or "special-need devices" (such as custom interventional devices developed by customers
themselves). We provide a full-process solution from "needs analysis" to "finished product delivery," addressing the customer's pain point of
not knowing how to perform dip coating.
Design Requirement | Process Solution | Final Effect | Applicable Device Examples | Process Challenges |
|---|---|---|---|---|
Complex Pattern Coverage | Adjust dipping speed (slow dipping ensures pattern gaps are filled) and curing temperature (graded curing prevents pattern deformation) | Coating uniformly covers bumpy patterns and mesh structures on the device surface, with no missed spots or accumulation | Guidewire handles with anti-slip patterns, mesh vascular stents, drainage catheters with multiple side holes | Requires precise control of dipping rate to avoid residual bubbles in deep patterns causing coating voids |
Differentiated Thickness Coating | Adopt "multiple dipping + local masking" process: areas needing thick coating are dipped multiple times, areas needing thin coating are protected with high-temperature resistant masking film | Coating thickness adjusted as needed on different parts of the same device (e.g., thin coating at catheter tip for flexibility, thick coating in middle for strength) | Cardiovascular catheters (tip needs flexibility, middle needs kink resistance), endoscopic biopsy forceps (thin coating on jaws for opening/closing, thick coating on shaft for rigidity) | Masking film must tightly adhere to device surface to avoid residual glue marks after curing |
Irregular Structure Adaptation | For curved, bifurcated, multi-lumen devices, use "rotational dipping" (ensures inner wall uniformity) and "local spraying + dipping combination" process | Coating covers without dead angles, consistent thickness at irregular parts (e.g., catheter bifurcations), without affecting device movement | Bifurcated vascular stents, multi-lumen drainage catheters, orthopedic instruments for joint areas | Need custom dipping fixtures based on device morphology to avoid device deformation during dipping process |
From selecting the right materials to adding features, from customizing form factors to providing comprehensive service, the customized capabilities of medical dip coating are, in essence, a technological extension centered on clinical needs. As interventional medicine evolves toward more sophisticated, minimally invasive, and long-lasting treatments, customized dip coating will further push the boundaries of materials and processes, empowering more innovative medical devices with enhanced performance, and propelling medical technology to new heights.