FDA grants Suzhou CH Biomedical approval to begin clinical trials for the BrioVAD™ left ventricular assist system, promising innovative solutions for heart failure.


Recently, Suzhou CH Biomedical, Inc. announced that its independently developed next-generation fully magnetically levitated left ventricular assist system, BrioVAD™, has received approval from the U.S. FDA to conduct clinical trials. The milestone clinical trial, named INNOVATE, has obtained Investigational Device Exemption (IDE) from the FDA, allowing conditional initiation of patient recruitment.
This study will evaluate the efficacy and safety of BrioVAD™, a new fully magnetically levitated left ventricular assist system developed by CH Biomedical, in treating refractory heart failure. Meanwhile, CH Biomedical has convened the first investigator meeting for the study and initiated related startup work for clinical trial centers in the United States.

CH Biomedical Holds First Investigator Meeting for INNOVATE Clinical Trial
The BrioVAD™ left ventricular assist system, approved by the FDA in the United States, builds upon the excellent blood compatibility of the existing product, CH-VAD. Through several technological innovations, it achieves portability of external carrying components while further enhancing overall system performance. Additionally, the INNOVATE study will employ a randomized controlled design to compare and evaluate BrioVAD™ with previously FDA-approved products.
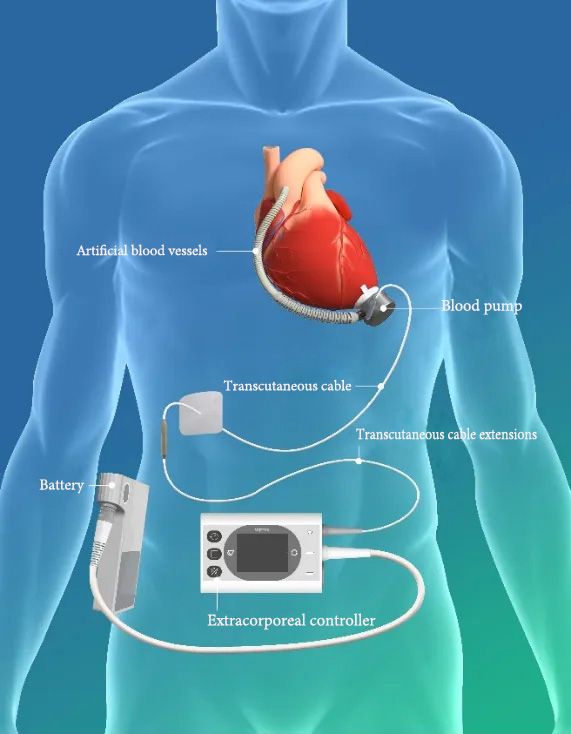
Left Ventricular Assist System BrioVAD™ Product Schematic
CH-VAD is the first domestically developed fully magnetically levitated implanted left ventricular assist device (LVAD) in China. Developed jointly by the team at Fuwai Hospital and CH Biomedical, it weighs 186g, with a blood pump diameter of 50mm, thickness of 26mm, and maximum flow rate of 10L/min. The core technology of this product mainly involves fully magnetically levitated blood pump technology, which has obtained multiple patents in China and the United States, making it a domestically pioneered medical device. Compared to similar international products, its key performance indicators have reached the same level, with smaller pump size and better implant invasiveness.
CH-VAD has been clinically available since 2018 and received NMPA approval for use with specific artificial blood vessels in 2021, providing mechanical support for the blood circulation of end-stage refractory heart failure patients, used as transitional therapy before heart transplantation or to recover heart function.

CH® Implantable Left Ventricular Assist System (CH-VAD)
CH-VAD consists of internal implant components, external carrying components, peripheral components, and specialized surgical tools. It is an electromechanical integrated device used to partially replace heart pumping function and maintain human blood circulation. Its core component is a blood pump that draws blood from the heart, increases its pressure, and delivers it to the aorta, unloading the natural heart's workload, allowing it to rest, while also supplementing insufficient natural heart pumping capacity.
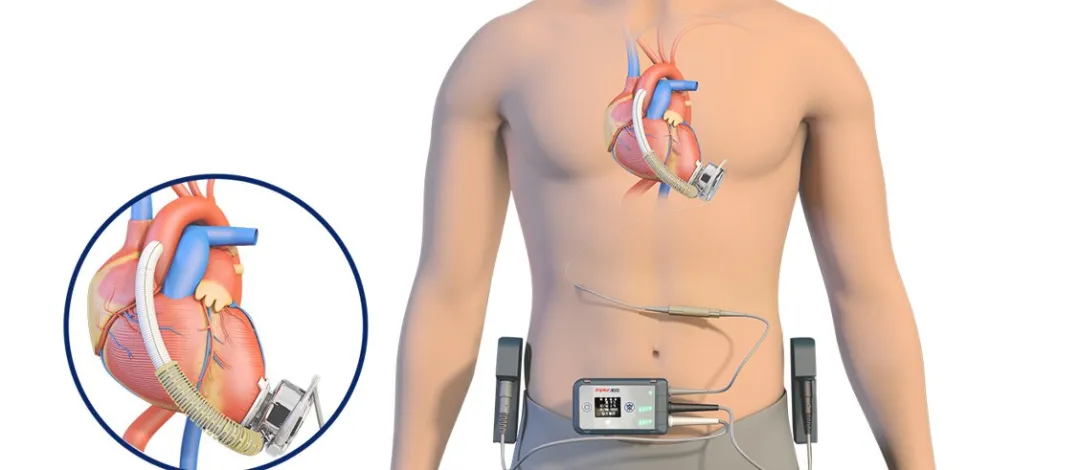
Since its launch, CH-VAD has been used in over 180 patients in more than 40 top cardiac hospitals nationwide. The first patient has survived for over 6 years and continues to lead a high-quality life. CH-VAD has a complete independent intellectual property rights system built from the ground up and has obtained patent protection in China, the United States, Europe, Japan, and other countries and regions.
In April 2024, at the ISHLT's "Spin Doctors and Heart Hotties: Unleashing the Future of MCS Devices" symposium, Professor Xianqiang Wang from Fuwai Hospital, Chinese Academy of Medical Sciences, presented the long-term follow-up results of CH-VAD on the international stage. In this single-center, retrospective, observational study, end-stage heart failure patients treated with CH-VAD showed high survival rates and low complication rates, with no instances of pump thrombosis, disabling strokes, or major device failures.
Research Objectives
To further evaluate the long-term efficacy and safety of CH-VAD, Fuwai Hospital conducted this single-center, retrospective, observational study, including heart failure patients treated with CH-VAD from June 2017 to August 2023, to statistically analyze their clinical data and long-term prognosis.
Research Methods
This study included 50 patients with follow-up durations ranging from 3 months to 6.7 years. The average duration of pump support for patients was 2.4 years, and all 50 patients successfully underwent LVAD implantation. Patients' blood pump operation was stable both intraoperatively and postoperatively, and hemodynamics returned to normal.
Research Results
Using Kaplan-Meier survival analysis, the 1-year, 2-year, and 3-year survival rates of patients were 93%, 93%, and 89%, respectively, all higher than those in international clinical studies and real-world settings. Among the 50 patients, only 2 underwent heart transplantation bridging. Three patients had their heart function completely restored and LVAD removed.
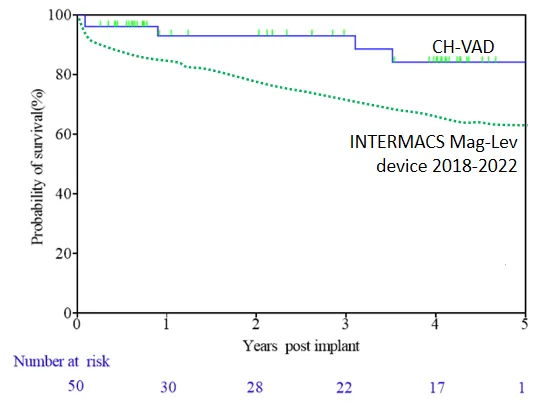
Patient survival during follow-up
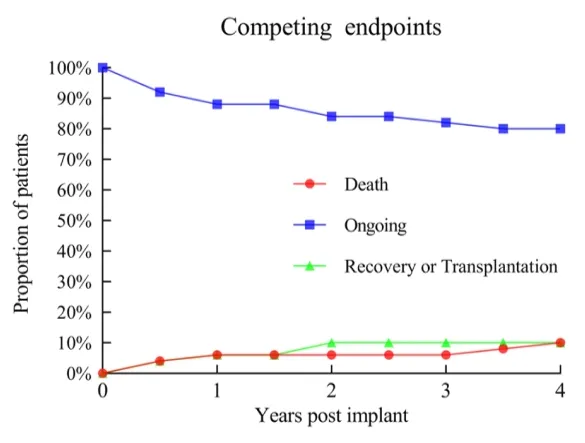
Incidence of events for different endpoints
In this study, the overall incidence rate of adverse events was low. The excellent blood compatibility of CH-VAD was reflected, with no observed instances of pump thrombosis, hemolysis, or disabling stroke events.
Among all patients, only 2 experienced ischemic strokes (0.02 per person-year), and 1 experienced a hemorrhagic stroke (0.01 per person-year), with all patients' neurological functions recovering during follow-up. The most common adverse event was postoperative infection, which was effectively prevented and controlled with the accumulation of interdisciplinary management experience, leading to good prognosis for the vast majority of patients. There were no major device failures or pump replacement surgeries during the follow-up period, indicating high long-term reliability of the device.
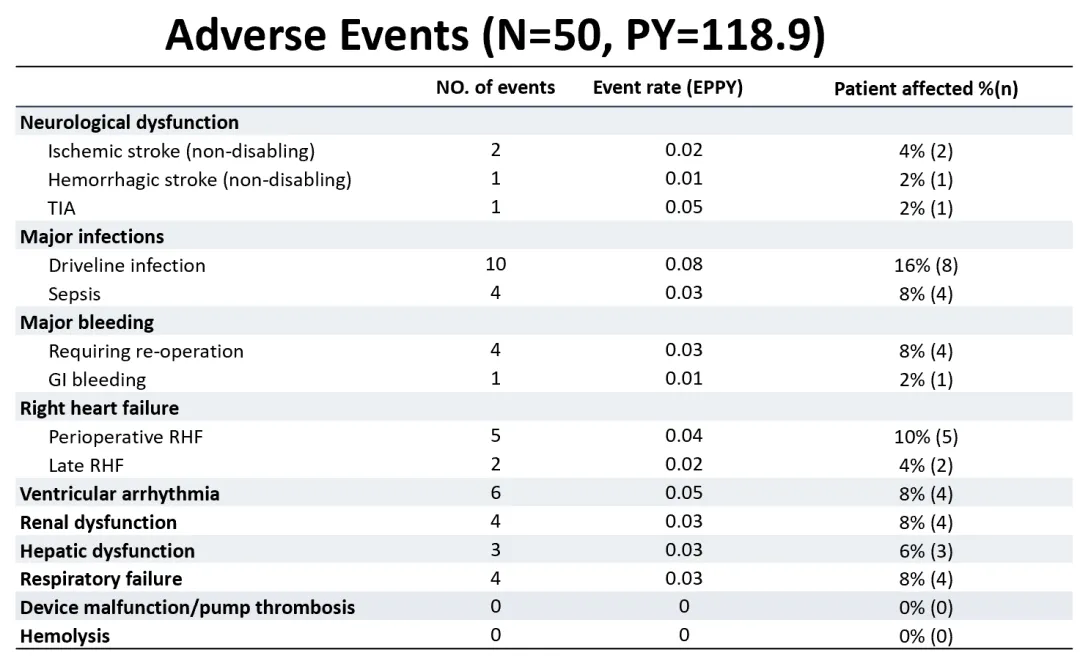
Incidence of various types of adverse events
On April 15, 2024, the U.S. Food and Drug Administration (FDA) announced a recall of the HeartMate II and HeartMate 3 implantable left ventricular assist systems by Abbott/Thoratec due to occurrences of Entrinsic Outflow Graft Obstruction (EOGO) caused by external forces. This recall affected 13,883 devices, accounting for 56% of the total market products, with a Kaplan Meier estimated occurrence rate of 0.24% at 2 years and 2.06% at 5 years for HeartMate 3 EOGO.
The EOGO incidents involving HeartMate II and HeartMate 3 in this recall are not new to the industry. Thus far, the FDA has received numerous adverse event reports regarding EOGO with HeartMate, totaling 273 injury events and 14 death events.
It's worth noting: Firstly, a Class I recall by the FDA for medical devices does not mean the cessation of production or use of the product, but rather corrective action is taken for the identified problems, and the devices will continue to be used in clinical settings. Secondly, the reason for the Class I recall of HeartMate II and HeartMate 3 by the FDA is EOGO, which is caused by the dense PTFE material reinforcement sheath of HeartMate II and HeartMate 3, resulting in the accumulation of exuded non-cellular biomaterial, forcing the artificial blood vessel to squeeze inward. However, domestic LVAD devices represented by CH-VAD have solved the above design defects of HeartMate through the hollow design of the reinforcement sheath.
Magnetic levitation technology enables stable rotation without the need for liquid conditions, significantly reducing blood damage, and thus reducing the occurrence of related complications such as thrombosis and stroke. CH-VAD achieves stable suspension in five degrees of freedom on the X, Y, and Z axes by arranging the motor stator and magnetic levitation stator on the inner and outer sides of the rotor, ensuring good shock resistance and stable long-term operation, reducing blood damage. Simultaneously, the optimized flow channel design ensures smooth blood flow from the inlet tube to the impeller position via a nasal cone, with a U-shaped secondary flow channel with a gap of 250μm, ensuring both smooth blood flow and good flushing effects.
Professor Zengsheng Chen from Beihang University once emphasized that the "culprit" of this recall lies in the dense PTFE material reinforcement sheath of HeartMate II and HeartMate 3, while domestic LVAD devices represented by CH-VAD have solved the problem of artificial vessel squeezing through the hollow design of the reinforcement sheath.
Today’s VADs are state-of-the-art implantable pumps. Take the CH-VAD, for example. It's equipped with a remarkable impeller, a single moving part that efficiently propels blood in a single direction. Suspended by magnets, this impeller operates without friction and has no components prone to wear and tear.
Impellers play a crucial role in VADs. When current passes through the magnets, the impeller begins to spin. Blood is drawn into the impeller's center under negative pressure, and its rotating vanes swiftly accelerate the blood from the center to the outer edges. This transformed blood, now under positive pressure, flows out of the pump through the discharge nozzle into the outflow cannula.
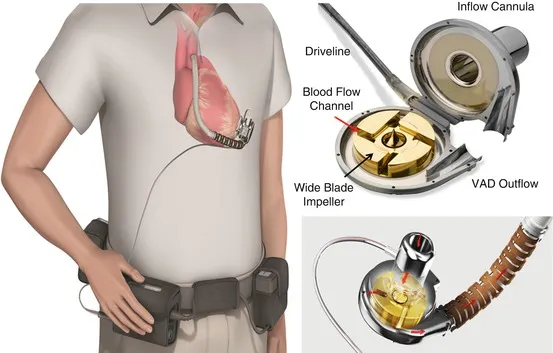
The design of the impeller is paramount. Held in place by magnets and specialized bearings, it spins smoothly without touching the sides. This design not only minimizes damage but also ensures the device's longevity.
At Manners Technology, we specialize in crafting parts for medical devices, including impellers for VADs. Our commitment to quality is evidenced by our certifications, including ISO 9001:2015 and ISO 13485.
We're also collaborating with a leading German company to develop even more efficient and reliable impellers for VADs. Together, we strive to produce parts that exceed expectations. Whether you require specialized parts or assistance with designs, our customized services are tailored to meet your needs.