Discover the Ti-Force Lift Double Button implant, designed for precise ligament reconstruction and tension adjustment with biocompatible titanium and UHMWPE sutures.


The Ti-Force Lift Double Button is an implant designed for ligament reconstruction and tension adjustment. Its uniqueness lies in the use of two biocompatible titanium buttons, combined with ultra-high-molecular-weight polyethylene (UHMWPE) surgical sutures, to support adjustable tension. It is commonly used in the repair of acromioclavicular joint ligaments, and depending on surgical indications, it can also be utilized in ligament repairs for important joints such as the wrist, ankle, and knee. Manners Technology, with years of expertise in medical products, keeps pace with innovative medical designs and continuously updates equipment, offering unique solutions for acromioclavicular joint surgery and syndesmosis surgery.

Ti-Force Lift Double Button, as a specialized implant for acromioclavicular joint surgery, demonstrates unique advantages in acromioclavicular joint reconstruction procedures. The clever combination of two biocompatible titanium buttons and UHMWPE surgical sutures not only makes the surgery more convenient but also provides the feature of adjustable tension, offering surgeons greater flexibility during the procedure. This innovation provides significant convenience for ligament repairs, which play a crucial role in plastic and reconstructive surgery, allowing for optimal fixation. Ti-Force Lift Double Button becomes an ideal choice for surgeries on key joints such as the wrist, ankle, and knee, where adjustable tension is desirable.
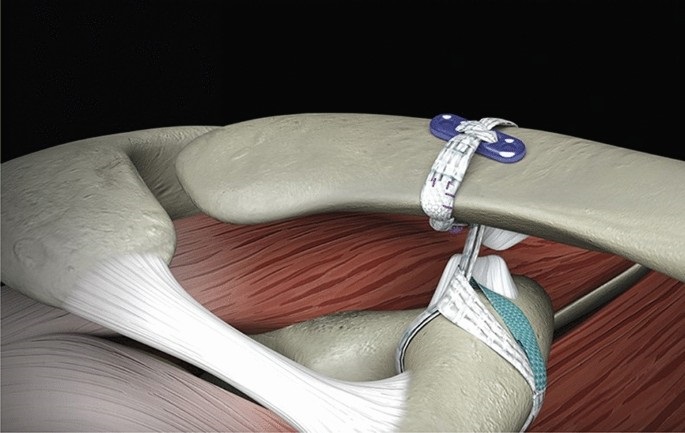
In the treatment of acromioclavicular joint lesions, Ti-Force Lift Double Button has found widespread application, especially in surgeries addressing acromioclavicular joint ligament ruptures. It provides surgeons with a highly adjustable and biocompatible option to meet the needs of different patients and surgical scenarios. Its reliable and durable treatment outcomes offer patients a better path to recovery.
Apart from its application in acromioclavicular joint surgery, Ti-Force Lift Double Button also demonstrates outstanding performance in syndesmosis surgery. Through the synergistic action of two titanium buttons and UHMWPE sutures, this implant not only ensures the success of the surgery but also provides surgeons with the opportunity to fine-tune tension, catering to the needs of different patients.
In summary, Ti-Force Lift Double Button represents Manners Technology’s commitment to continuous innovation in the field of orthopedic implants. By continually enhancing product design and material craftsmanship, Manners provides surgeons with more advanced and reliable tools to achieve better treatment outcomes, ensuring patients can return to normal life quickly and safely.
Ti-Force Lift Double Button, as a widely used orthopedic implant for the shoulder, incorporates two biocompatible titanium buttons to complement UHMWPE surgical sutures. Manners Technology utilizes advanced Computer Numerical Control (CNC) technology for precise machining, especially in the design with a self-locking system.

Firstly, in terms of material selection, Ti-Force Lift Double Button uses high-quality titanium alloy sheets. Titanium alloy boasts excellent biocompatibility and mechanical properties, making it suitable for orthopedic implants and ensuring that patients do not experience rejection or other adverse reactions post-surgery. During the manufacturing process, Manners employs a one-time core-walking machine for precision machining. This advanced machining technology offers high precision and stability, with error control within ±0.01mm. Initially, outer shapes are cut to ensure uniformity in the appearance of each Ti-Force Lift Double Button. Subsequently, hole machining is carried out, with stricter requirements due to the need for holes to pass through suture lines. This guarantees precise diameters and positions for each hole to meet the practical application requirements of the implant.
High precision machining ensures that each part of the implant complies with design specifications, enabling it to securely lock in place once adjusted to the correct position, preventing unnecessary movement or loosening and enhancing its performance and reliability. This contributes to providing stable bone-implant integration, supporting patient recovery and healing. Similarly, for implants like Ti-Force Lift Double Button, which require fine-tuning and adjustable design, it is crucial to ensure that these functionalities can be reliably implemented during surgery.
To enhance implant comfort and the patient’s surgical experience, the surface of Ti-Force Lift Double Button needs to be smooth. After machining is completed, the product undergoes surface treatment to ensure a defect-free surface without burrs or other imperfections. This not only helps reduce friction between the implant and surrounding tissues but also improves the patient’s surgical recovery experience. Overall, the manufacturing process of Ti-Force Lift Double Button is precise and rigorous, taking into consideration material selection and control of the manufacturing process.
Following manufacturing, a series of biomechanical tests are necessary to verify the structural strength and stability of Ti-Force Lift Double Button. This includes durability tests, load tests, etc., ensuring that the implant can maintain its design performance under various stresses. Manners Technology is committed to providing high-quality orthopedic implant products to patients and healthcare professionals through advanced technology and excellent craftsmanship. Ti-Force Lift Double Button represents the latest technology and a high level of craftsmanship in the medical industry, offering a reliable and highly personalized treatment option for patients.