Gain insights into Medtronic's Aurora EV-ICD system & how ICDs are made. It monitors heart rhythms & delivers shocks when needed, improving therapy & patient comfort.


Earlier this year, the FDA cleared Medtronic Aurora EV-ICD system. It incorporates the company's MRI SureScan technology and Epsila EV MRI SureScan lead. The Aurora EV-ICD system is designed to allow lead placement below the sternum, outside of the heart and veins.
Your doctor may suggest an implantable cardioverter defibrillator (ICD) if you have certain heart problems. While the idea of a device that shocks your heart may cause anxiety, ICDs can restore normal heart function and potentially save your life.
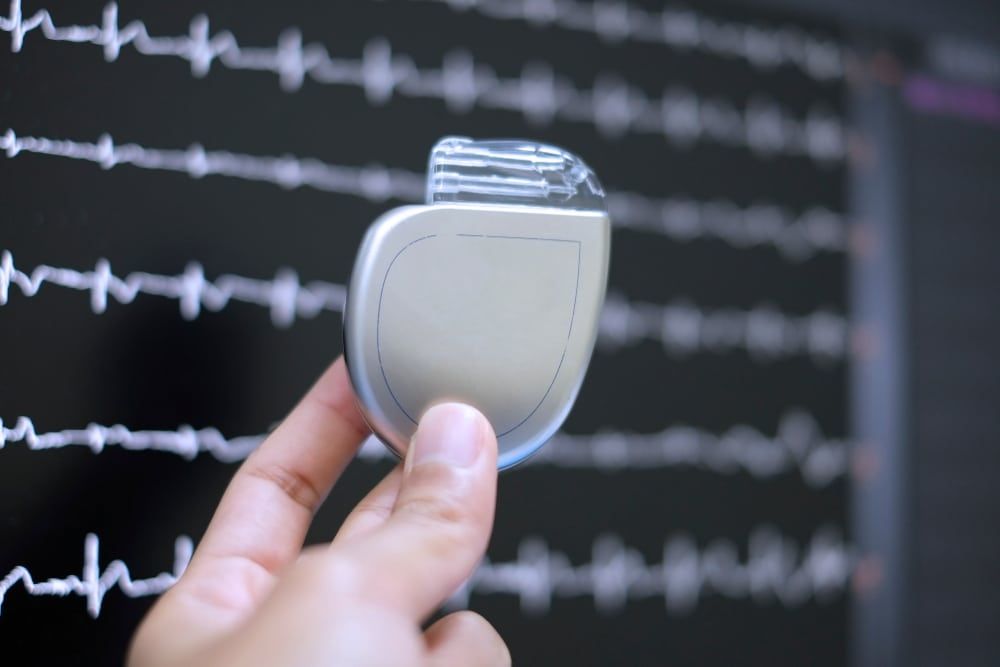
An ICD is an implantable cardioverter defibrillator. It is a small, battery-powered device that is surgically implanted beneath the skin, typically in the chest area. The device continuously monitors the heart's rhythm using thin wires that connect to the heart.
Its primary function is to safeguard individuals with heart rhythm irregularities. It detects abnormal heart rhythms, such as ventricular tachycardia or ventricular fibrillation. Then, it delivers precisely timed electric shocks to restore the heart's rhythm to a normal and healthy pace. The device serves as a vigilant guardian, swiftly responding to irregularities to maintain optimal heart function and prevent life-threatening situations.
Medtronic's Aurora EV-ICD system aims to find the perfect solution. It should keep the leads out of the heart and vasculature while providing all the therapies available in modern transvenous systems. This includes pacing, low-energy defibrillation, small device size, and modern system longevity.
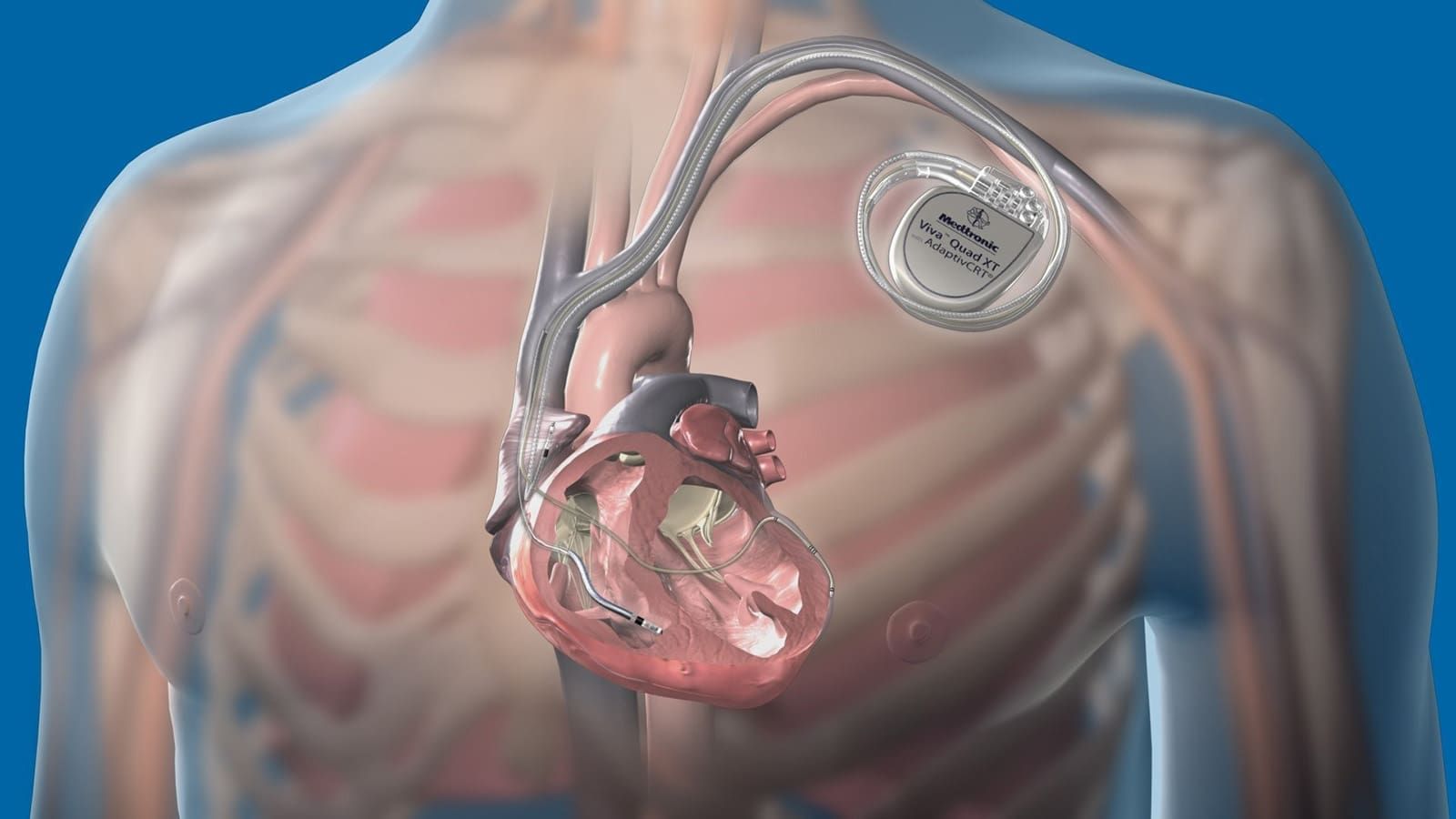
To achieve this, Medtronic introduces a new paradigm for defibrillation and implantation. Medtronic's team demonstrated that the system can be implanted in the substernal space, which is the area between the heart and the sternum.
The Aurora EV-ICD system combines features transvenous ICDs with additional advantages not offered by subcutaneous ICDs. It includes Anti-tachycardia pacing (ATP) to terminate ventricular arrhythmias, Pause Prevention Pacing to address brief heartbeat pauses, and a 40 Joule Defibrillation Energy capability in a compact device size. The system has Medtronic's exclusive PhysioCurve design, which enhances patient comfort and implant acceptance. It is projected to last 11.7 years, minimizing the need for device replacement procedures over a patient's lifetime.
The implantable cardioverter defibrillator (ICD) consists of a small can housing electronics, a lithium battery, and high-voltage capacitors for shocks, along with soft insulated wire leads. In a short, It has three main accessories as follows:
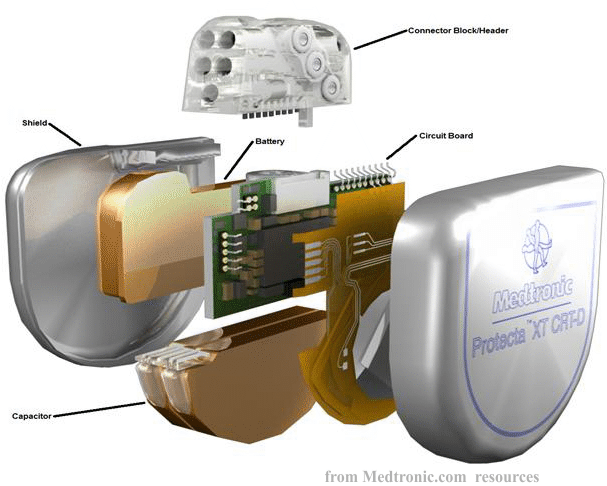
The ICD generator comprises essential components such as the battery and electronic circuitry, including the capacitor, which coordinates pacing, sensing, and defibrillation functions. The battery's lifespan typically ranges from 4 to 7 years depending on device usage. Circuitry within the ICD governs the delivery of pacing and anti-tachycardia therapies, offering a range of programming options tailored to individual needs. Capacitors store energy for defibrillation, allowing for the release of electrical energy across the heart.
The ICD communicates with the heart via ventricular defibrillator leads, often supplemented with atrial and LV leads in specific models. The leads are connected to the ICD through a header, which secures them in place with set screws. The metal casing of the ICD, known as the "can," safeguards its electrical components from fluids and external electrical sources.
Ventricular defibrillator leads are constructed with multiple internally separated metal wires encased in insulation, facilitating the transmission of pacing/sensing signals and the delivery of shocks. These leads typically feature one or two coils, with shocks delivered between the coil(s) and potentially the ICD can. Each lead has its pin for connection to the ICD header, enabling both pacing/sensing and defibrillation functions.
Manners offers a wide range of metal manufacturing services, including titanium alloys, stainless steel, and more. For instance, we can manufacture the metal casing of the pulse generator in ICD, as well as the metal scaffold in the connector module. Additionally, we provide comprehensive processes such as laser cutting, laser welding, surface treatment, coating services, etc.
An ICD, similar to a pacemaker, consists of a pulse generator and leads. The pulse generator is made of titanium, the connector module is made of rigid polyurethane, the leads are insulated with soft polyurethane or silicone, and the electrodes are made with platinum or platinum-plated metals.
While epoxy resin and stainless steel were used in earlier manufacturing of pulse generators’ shields, titanium has become the predominant choice today due to its superior biocompatibility and biostability. It offers better protection because of its lightweight yet robust nature compared to stainless steel. Manners specializes in manufacturing high-quality titanium products. The majority of our orthopedic surgical instruments, such as anchors, interference screws, spine posterior, and knee arthroscopic implant, are made from titanium metal.
These shields are welded shut to prevent moisture ingress and are further enhanced with Parylene coating, ensuring optimal performance and longevity within the human body. Manners possesses cutting-edge 5-axis laser welding systems and employs skilled laser engineers, facilitating tailored seamless welding processes that minimize heat and yield uniform welds, free from warping or part discoloration.
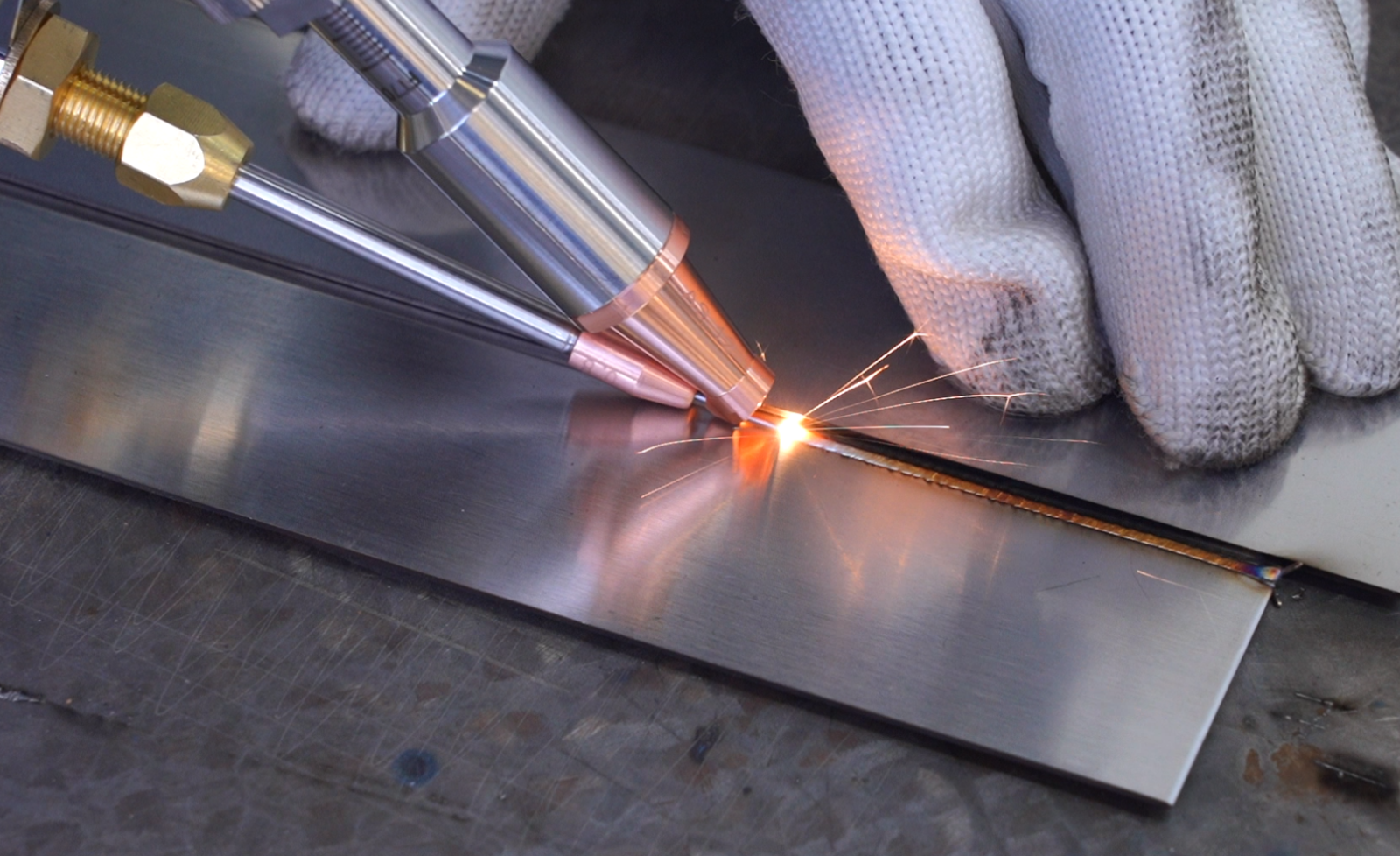
The connector module is the component that physically and electrically connects the pulse generators to the leads. Typically, the module comprises a stainless steel scaffold integrated into a polymeric block, commonly crafted from rigid polyether polyurethane or epoxy.
Ensuring proper bonding between the connector module and the pulse generator atop feedthroughs is essential, facilitated by meticulous surface cleaning and treatment to promote adhesion, an aspect where Manners Technology excels with their advanced surface treatment technologies.
Manners provides two types of surface treatment services: Electropolishing and Ultrasonic cleaning. Electropolishing is an electrochemical finishing method that selectively removes a thin layer of material from metal parts, usually stainless steel or similar alloys. This meticulous process results in a glossy, smooth, and exceptionally clean surface finish.
However, for precision instruments like ICD intended for implantation into the human body, there are typically higher requirements, necessitating the use of ultrasonic cleaning. Ultrasonic cleaning operates by transmitting high-frequency sound waves through a liquid to thoroughly scrub the surface of immersed parts. Typically at 40 kHz, these sound waves agitate the liquid solution of water or solvent, and cause the cavitation of solution molecules to induce the dirt layer to be dispersed, emulsified, and stripped to achieve the cleaning purpose.

The metal scaffold, crucial for connecting wires from the feedthroughs to the electrodes of inserted leads, is expertly fabricated by Manners using high-quality 5-axis CNC technology. This technology ensures precise and reliable performance, improving efficiency by handling all milling operations for complex geometries in a single setup. With the ability to machine different sides of a workpiece without repositioning, 5-axis machines reduce operational costs and errors while offering high surface finish accuracy. Additionally, their high-speed operation with minimal noise results in enhanced surface finish and reduced post-processing expenses, ultimately boosting overall machining efficiency.
In Manners Technology, our products are inspected for burrs, cracks, pits, machining marks and dirt. Each process is cleaned and inspected before moving on to the next in Manners. Please call or email for pricing and lead-time on our standard stock or to request a quotation for custom dimensions.